Alpha Sophia for Contract Research Organizations (CROs)

Challenge
Contract Research Organizations (CROs) and Contract Development and Manufacturing Organizations (CDMOs) face unique challenges in identifying healthcare providers (HCPs) and healthcare organizations (HCOs) that meet stringent requirements for clinical trial and development projects. This process involves selecting candidates with the right patient demographics, therapeutic expertise, and infrastructure to ensure trial success. Additionally, CDMOs often need partners capable of managing complex manufacturing demands. Navigating vast datasets to locate HCPs and HCOs with the necessary experience, compliance certifications, and geographical relevance adds complexity. Ensuring regulatory compliance across diverse regions further complicates site selection and investigator recruitment.
Solution
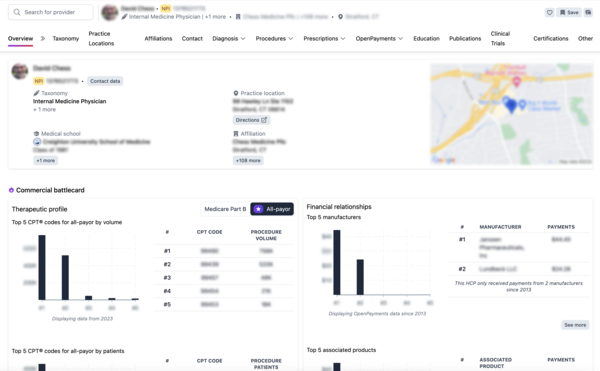
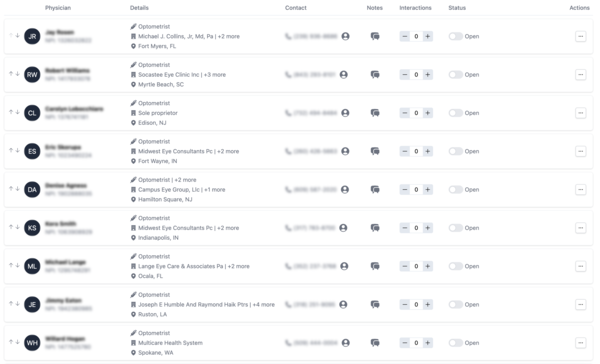
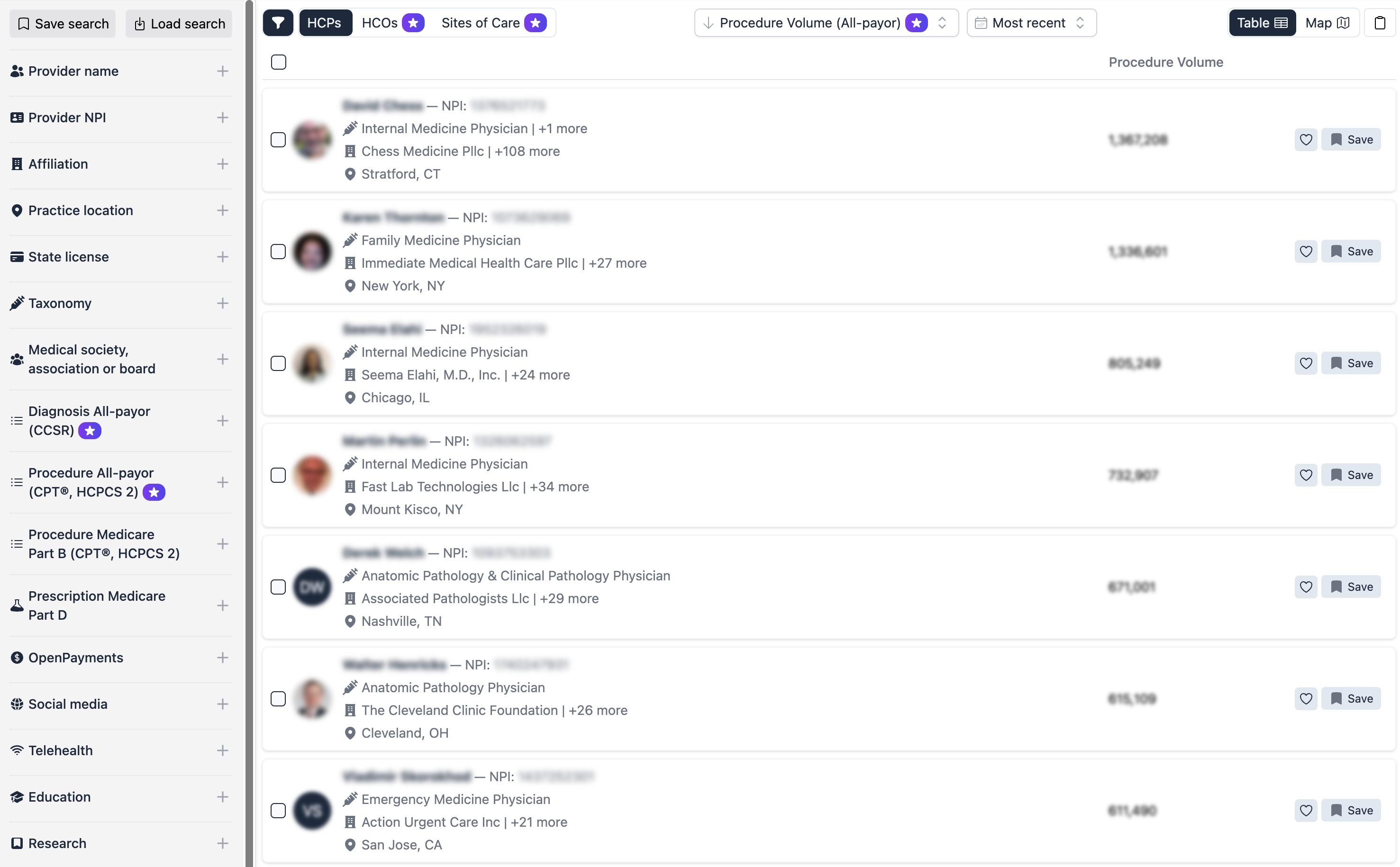
Alpha Sophia empowers CROs and CDMOs to optimize their recruitment and site selection processes with advanced filtering capabilities tailored to research, clinical, and manufacturing needs. By filtering HCPs and HCOs based on research contributions, patient demographics, therapeutic expertise, affiliations, and regulatory credentials, Alpha Sophia helps organizations identify ideal trial sites, investigators, and partners. These precise filters allow CROs and CDMOs to focus on high-quality, compliant locations and experts, ensuring trials and manufacturing processes are conducted efficiently and meet regulatory standards. This targeted approach enhances the quality, speed, and success rate of clinical trials and development projects, driving superior outcomes for clients.